假白欖烷型大環二萜類化合物及其應用的制作方法
本發明一類新發現的天然化合物及其應用,特別涉及假白欖烷型大環二萜類化合物及其應用。
背景技術:
多藥耐藥(MDR)是由一種藥物誘發,導致對其他結構和作用機制不同的藥物同時產生交叉耐藥的現象。MDR是導致腫瘤化療失敗的重要原因之一。MDR的一個主要作用機制為P-糖蛋白(Pgp)在腫瘤細胞膜上的過表達。Pgp是種ATP轉運酶,能夠對抗腫瘤藥主動跨膜轉運,起到藥物外排泵的作用,從而減少抗腫瘤藥物在體內的濃度。目前針對腫瘤MDR藥物研發最為普遍的做法是尋找高效、低毒、具有良好藥物代謝動力學行為的Pgp抑制劑。研究證實熒光染料羅丹明123在細胞內的蓄積與Pgp糖蛋白功能表達呈負相關,進一步證實了Pgp糖蛋白的排藥泵作用,將Rho 123與細胞作用后,通過檢測待篩選藥物存在/不存在的情況下細胞內熒光的蓄積量或檢測細胞外熒光的排出量,可篩選出Pgp糖蛋白抑制。迄今為止,雖然有大量的Pgp抑制劑被研究報道,但仍未見以Pgp為靶點的抗腫瘤藥物上市。咎其原因在于毒性、結合親和力、及藥動學等缺陷。例如,第一代藥物例如維拉帕米、環孢霉素A等,與P-gp的結合親和力較弱,且因缺乏特異性,易與細胞中其他組織蛋白或酶發生相互作用,產生不良的藥動學效應。第二代藥物如valspodar,elacridar,biricodar等,對CYP3A4具有抑制作用,影響抗腫瘤藥物代謝,從而導致后者毒副作用增大。第三代多藥耐藥抑制劑像tariquidar(XR9576),zosuquidar(LY335979)由于不良反應止步于臨床III期。
天然產物因具有結構多樣性和良好的生物兼容性是新藥研發的重要來源。文獻調研顯示大戟科植物中富含結構新穎的假白欖烷型二萜,該類二萜在細胞水平顯示出良好的Pgp抑制活性。然而,對該類二萜深入的藥效學、構效關系、機制研究尚且匱乏。
技術實現要素:
本發明的目的在于提供假白欖烷型大環二萜類化合物及其應用。
本發明所采取的技術方案是:
假白欖烷型大環二萜類化合物,其結構通式如式Ⅰ所示:
式中:R1,為苯甲酰氧基,煙酰氧基,或與C-2上的氫消除形成C2=C3雙鍵;R2,為氫或與R1同時消除形成C3=C4雙鍵;R3,為羥基,羰基,與R9形成氧橋,或與R4同時消除形成C5=C6雙鍵;R4,與R5同時消除形成C6=C7雙鍵;R5,為β-乙酰氧基,β-苯甲酰氧基,羰基,或為連接C6=C7雙鍵的乙酰氧基;R6,為氫或者乙酰氧基;R7,為氫,與R8形成三元環氧,與R8同時消除形成C11=C12雙鍵;R8,為氫,羥基,與R9形成三元環氧,或與R9同時消除形成C12=C13雙鍵;R9,為α-羥基,或與C-20上的氫消除形成C13=C20雙鍵;R10,為氫,羰基,為α-羥基,α-乙酰氧基或α-苯甲酰氧基;R11,為氫或乙酰氧基;R12,為羥基,羰基,乙酰氧基,對甲基苯磺酰氧基,對溴苯甲酰氧基,噻吩酰氧基或呋喃酰氧基。
作為上述化合物的進一步改進,R1為酰氧基時,R12為羥基,乙酰氧基,對甲基苯磺酰氧基,對溴苯甲酰氧基,吩酰氧基或呋喃酰氧基。
作為上述化合物的進一步改進,R1為苯甲酰氧基,R12為酰氧基時,R3與R9形成氧橋,或與R4同時消除形成C5=C6雙鍵。
作為上述化合物的進一步改進,化合物具體為:
YPTZ-1:(1S,2R,8R,9S,14R,15S)-1,8,9,14,15-五(乙酰基)-7-羰基-假白欖烷-3Z,5E,12E-三烯烴;YPTZ-2:(1S,2R,3S,4S,5S,8R,9S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-煙酰基-5-羥基-假白欖烷-6E,12E-二烯;YPTZ-3:(1S,2R,3S,4S,5S,8R,9S,12S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-5,12-二(羥基)-假白欖烷-6E,13(20)-二烯;YPTZ-4:(1S,2R,3S,4S,7S,8R,9S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-假白欖烷-5E,12E-二烯;YPTZ-5:(1S,2R,3S,4R,5S,8R,9S,11R,12R,13R,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-5,13,11,12-二(醚基)-假白欖烷-6E-烯;YPTZ-6:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-7,9,15-三(乙酰基)-3-苯甲酰基-1,13,14-三(羥基)-假白欖烷-5E,11E-二烯;YPTZ-7:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3,4-二(苯甲酰基)-1,7,13-三(羥基)-假白欖烷-5E,11E-二烯;YPTZ-8:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9-乙酰基-3,7-二(苯甲酰基)-1,13,14,15-四(羥基)-假白欖烷-5E,11E-二烯;YPTZ-14:(1S,2R,3S,4S,7R,9R,13R,14R,15S)-1,3,7,9,13,14,15-七(羥基)-假白欖烷-5E,11E-二烯;YPTZ-15:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-3-苯甲酰基-1,7,9,13,14,15-六(羥基)-假白欖烷-5E,11E-二烯;YPTZ-16:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-3,7-二(苯甲酰基)-1,9,13,14,15-五(羥基)-假白欖烷-5E,11E-二烯;YPTZ-17:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-1,9,15-三(乙酰基)-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-18:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-1,9,15-三(乙酰基)-3,7-二(苯甲酰基)-1,13-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-19:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1-對甲基苯磺酰基-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-20:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1-對溴苯甲酰基-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-21:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1-呋喃酰基-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-22:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1-噻吩酰基-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-23:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1,3,7-三(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-24:(4S,9R,13R,14R,15R)-9,15-二(乙酰基)-13,14-二(羥基)-假白欖烷-2Z,5E,11E-三烯;YPTZ-25:(1S,2R,3S,4S,7R,9R,13R,15S)-1,9,15-三(乙酰基)-3,7-二(苯甲酰基)-13-羥基-14羰基-假白欖烷-5E,11E-二烯;YPTZ-26:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3,7-二(苯甲酰基)-1,13,14-三(羥基)-假白欖烷-5E,11E-烯;YPTZ-27:(1S,2R,8R,9S,14R,15S)-9,15-二(乙酰基)-1,8,14-trihydroxy-7-羰基-假白欖烷-3Z,5E,12E-三烯;YPTZ-28:(1S,2R,8R,9S,14R,15S)-1,9,14,15-四(乙酰基)-8-羥基-7-羰基-假白欖烷-3Z,5E,12E-三烯;YPTZ-29:(1S,2R,3S,4S,5S,8R,9S,14R,15S)-7,8,9,14,15-五(乙酰基)-1,3-二(苯甲酰基)-5-羥基-假白欖烷-6E,12E-二烯;YPTZ-30:(1S,2S,3S,4S,5S,8R,9S,14R,15S)-二(乙酰基)-3-苯甲酰基-1,5,9,14,15-五(羥基)-假白欖烷-6E,12E-二烯;YPTZ-31:(1S,2R,3S,4S,8R,9S,12S,13R,14R,15S)-1,8,9,14,15-五(乙酰基)-3-苯甲酰基-12,13-醚-7-羰基-5E-烯;YPTZ-32:(1S,2R,3S,4S,8R,9S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-5-羰基-假白欖烷--6E,12E-二烯;YPTZ-33:(1S,2R,3S,4S,8R,9S,12S,13R,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基12,13-醚-假白欖烷-6E-烯;YPTZ-34:(1S,2R,3S,4R,5S,8R,9S,12S,13S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-12-羥基-5,13-醚-假白欖烷-6E-烯;或YPTZ-35:(1S,2R,3S,4R,5S,8R,9S,12S,13S,14R,15S)-1,7,8,9,12,14,15-七(乙酰基)-3-苯甲酰基-5,13-醚-假白欖烷-6E-烯。
一種治療腫瘤的組合物,包括對腫瘤具有治療作用的活性成分和增效成分組成,增效成分為好述的假白欖烷型大環二萜類化合物,或為:
YPTZ-9:(1S,2R,3S,4S,5S,8R,9S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-5-羥基-假白欖烷-6E,12E-二烯;YPTZ-10:(1S,2R,3S,4S,5S,8R,9S,14R,15S)-7,8,9,14,15-五(乙酰基)-3-苯甲酰基-1,5-二(羥基)-假白欖烷-6E,12E-二烯;YPTZ-11:(1S,2R,3S,4S,7S,8R,9S,14R,15S)-1,7,8,9,14,15-五(乙酰基)-3-苯甲酰基-7-羰基-假白欖烷-5E,12E-二烯;YPTZ-12:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3,7-二(苯甲酰基)-1,13,14-三(羥基)-假白欖烷-5E,11E-二烯;YPTZ-13:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3-苯甲酰基-1,7,13,14-四(羥基)-假白欖烷-5E,11E-二烯中的至少一種。
作為上述組合物的進一步改進,對腫瘤具有治療作用的活性成分選自博來霉素、更生霉素、紅必霉素、阿霉素和黃膽素,以及植物類抗癌藥紫杉醇、長春堿、長春新堿、三尖杉酯堿。
假白欖烷型大環二萜類化合物的制備方法,包括如下步驟:
1)將紅雀珊珊全草粉粹進行醇提,得浸膏;
2)依次使用石油醚和乙酸乙酯萃取浸膏,隨后石油醚萃取物經MCI柱層析,分別用30%、50%、70%、90%、100%甲醇-水梯度洗脫,得各種階段的洗脫液;
3)將洗脫液,通過硅膠柱層析、凝膠柱層析、ODS柱層析或高效液相色譜中的至少一種分離,得到假白欖烷型大環二萜類化合物或其前體;
對前體進行修飾,得到假白欖烷型大環二萜類化合物
作為上述制備方法的進一步改進,醇提為95%乙醇超聲提取3次。
本發明的有益效果是:
本發明的假白欖烷型大環二萜類化合物可以顯著地抑制Pgp活性,在細胞水平和動物水平表現出強于第三代藥物tariquidar的活性及安全性,有望成為對抗腫瘤耐藥的候選藥物。
本發明的假白欖烷型大環二萜類化合物同樣可作為高效Pgp活性抑制劑使用。
附圖說明
圖1是化合物的分離流程圖;
圖2是假白欖烷型二萜對羅丹明123外排量效關系活性篩選結果;
圖3是YPTZ-26在肝微粒體中的代謝曲線;
圖4是抗異位移植腫瘤的體內研究(A)異位移植的腫瘤體積(B)異位移植腫瘤的裸鼠體重(C)異位移植腫瘤的裸鼠生存率(D)對照組和聯合用藥組的腫瘤重量(E)存活異位移植腫瘤的裸鼠及其腫瘤。
具體實施方式
發明人從大戟科植物紅雀珊珊中分離得到8個全新的假白欖烷型大環二萜,并對部分化合物進行結構修飾獲得22個新衍生物。將所得系列二萜進行多藥耐藥性相關的活性測試,發現了迄今為止活性最強的天然Pgp抑制劑,其中1個新化合物有望成為對抗腫瘤耐藥的候選藥物。
下面結合具體實施例進一步詳細說明本發明。除非特別說明,本發明采用的試劑、設備和方法為本技術領域常規市購的試劑、設備和常規使用的方法。
現在參考下列說明性的實施例,進一步解釋本發明,其中NMR波譜采用Bruker AM-400spectrometer記錄,TMS內標。柱色譜硅膠(300~400目):青島海洋化工廠;GF254硅膠薄層色譜預制板:青島海洋化工廠;MCI填料(CHP20P,75~150μm):日本Mitsubishi公司;葡聚糖凝膠(Sephadex LH-20):美國GE公司;ODS填料(12nm,S-50μm):日本YMC公司;其余溶劑和試劑:分析純(AR),天津市百世化工有限公司。
假白欖烷型大環二萜類化合物的制備
1)取紅雀珊珊全草1kg,粉碎成粗粉,加入6倍體積量的95%乙醇,浸泡一周,減壓抽濾,減壓回收乙醇,余液濃縮至相對密度為1.20的稠膏,重復三次以上浸泡提取步驟,最終得紅雀珊珊乙醇提取物48g;
2)將紅雀珊珊乙醇提取物用1L水溶解,依次用等量的石油醚、乙酸乙酯各萃取三次。合并石油醚萃取液,減壓濃縮,得到石油醚萃取物30g;
3)將石油醚萃取物應用MCI柱初步分段,以甲醇∶水=3∶7~10∶0洗脫;然后采用Sephadex LH-20(氯仿:甲醇=1:1)進行粗分離,再利用200-300目的柱色譜硅膠以石油醚:丙酮、石油醚:乙酸乙酯或氯仿:甲醇等溶劑體系梯度洗脫,再反復采用凝膠柱Sephadex LH-20、ODS柱純化,最后經半制備高效液相色譜手段在(乙腈:水)或(甲醇:水)條件下進一步純化,最終得到8個全新的單體化合物,具體分離流程參考圖1。
分離產物的鑒定:
實施例1:最后通過半制備HPLC分離純化得到化合物YPTZ-1:(1S,2R,8R,9S,14R,15S)-1,8,9,14,15-五(乙酰基)-7-羰基-假白欖烷-3Z,5E,12E-三烯烴,其結構及數據如下:
YPTZ-1:[α]25D-18.9(c 0.11,CHCl3)。UV(MeOH)λmax(logε)251(3.57),206(3.88)nm。IR(KBr)νmax2970,1744,1694,1371,1220,1029,765cm-1。HREIMS m/z 599.2470[M+Na]+(calcd for C30H40O11Na,599.2463)。
YPTZ-1核磁數據(400MHz,CDCl3)
實施例2:
通過正相硅膠柱(氯仿:甲醇,1:0~5:1),純化得到YPTZ-2:(1S,2R,3S,4S,5S,8R,9S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-煙酰基-5-羥基-假白欖烷-6E,12E-二烯,其結構及數據如下:
YPTZ-2:[α]25D-58.4(c 0.13,CHCl3)。UV(MeOH)λmax(logε)263(3.61),226(3.85)nm。IR(KBr)νmax3476,2933,1745,1429,1373,1234,754,611cm-1。HREIMS m/z 782.3013[M+Na]+(calcd for C38H49NO15Na,782.2994)。
YPTZ-2核磁數據(400MHz,Methanol-d4)
實施例3
通過正相硅膠柱(氯仿:甲醇,1:0~5:1),純化得到YPTZ-3:(1S,2R,3S,4S,5S,8R,9S,12S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-5,12-二(羥基)-假白欖烷-6E,13(20)-二烯,其結構及數據如下:
YPTZ-3:[α]25D-34.2(c 0.19,CHCl3)。UV(MeOH)λmax280(2.85),232(3.95)nm。IR(KBr)νmax3567,3520,2973,2937,1761,1370,1222,771,715cm-1。HREIMS m/z 797.3004[M+Na]+(calcd for C39H50O16Na,797.2991)。
YPTZ-3核磁數據(400MHz,CDCl3)
實施例4
用凝膠柱(氯仿:甲醇1:1)進行分離得到YPTZ-4:(1S,2R,3S,4S,7S,8R,9S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-假白欖烷-5E,12E-二烯,其結構及數據如下:
YPTZ-4:[α]25D+53.3(c 0.12,CHCl3).UV(MeOH)λmax(logε)273(3.06),239(3.57)nm.IR(KBr)νmax2973,1737,1369,1219,1033,713cm-1.HREIMS m/z 675.3137[M+Na]+(calcd for C39H50O14Na,675.3093)。
YPTZ-4核磁數據(400MHz,CDCl3)
實施例5
通過半制備HPLC(CH3OH/H2O,8:2,3mL/min)分離純化得到化合物YPTZ-5:(1S,2R,3S,4R,5S,8R,9S,11R,12R,13R,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-5,13,11,12-二(醚基)-假白欖烷-6E-烯,其結構及數據如下:
YPTZ-5:[α]25D-43.3(c 0.06,CHCl3);UV(MeOH)λmax(logε)273(2.67),231(3.87)nm;IR(KBr)νmax2982,1742,1369,1240,1067,1036,712cm-1;HREIMS m/z 795.2867[M+Na]+(calcd for C39H48O16Na,795.2835)。
YPTZ-5波譜數據(400MHz,CDCl3)
實施例6
通過半制備高效液相(MeOH/H2O,7:3,3mL/min)純化得到YPTZ-6:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-7,9,15-三(乙酰基)-3-苯甲酰基-1,13,14-三(羥基)-假白欖烷-5E,11E-二烯,其結構及數據如下:
YPTZ-6:mp 160-163℃;[α]25D+11.9(c 0.12,CHCl3);UV(MeOH)λmax(logε)264(3.05),234(3.87)nm;IR(KBr)νmax3514,2974,2932,1732,1447,1374,1271,1248,1110,757,712cm-1;HREIMS m/z 639.2789[M+Na]+(calcd for C33H44O11Na,639.2776)。
YPTZ-6核磁數據(400MHz,CDCl3)
實施例7
通過半制備高效液相純化得到YPTZ-7:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3,4-二(苯甲酰基)-1,7,13-三(羥基)-假白欖烷-5E,11E-二烯,其結構及數據如下:
YPTZ-7:[α]25D-70.0(c 0.10,CHCl3);UV(MeOH)λmax273(3.20),236(3.93);IR(KBr)νmax3633,3534,2981,1711,1350,1268,1253,1110,1025,712cm-1;HREIMS m/z 701.2968[M+Na]+(calcd for C38H46O11Na,701.2932)。
YPTZ-7核磁數據(400MHz,CDCl3)
實施例8
在石油醚:丙酮(9:1)溶液中重結晶純化得到YPTZ-8:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9-乙酰基-3,7-二(苯甲酰基)-1,13,14,15-四(羥基)-假白欖烷-5E,11E-二烯,其結構及數據如下:
YPTZ-8:[α]25D-20.0(c 0.07,CHCl3);UV(MeOH)λmax273(3.28),233(3.81);IR(KBr)νmax3471,2940,1714,1452,1366,1262,1244,708cm-1;HREIMS m/z 659.2853[M+Na]+(calcd for C36H44O10Na,659.2827)。
YPTZ-8核磁數據(400MHz,CDCl3)
以下實施例中用到的化合物YPTZ-9~YPTZ-13分別為:
YPTZ-9:(1S,2R,3S,4S,5S,8R,9S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-5-羥基-假白欖烷-6E,12E-二烯
YPTZ-10:(1S,2R,3S,4S,5S,8R,9S,14R,15S)-7,8,9,14,15-五(乙酰基)-3-苯甲酰基-1,5-二(羥基)-假白欖烷-6E,12E-二烯
YPTZ-11:(1S,2R,3S,4S,7S,8R,9S,14R,15S)-1,7,8,9,14,15-五(乙酰基)-3-苯甲酰基-7-羰基-假白欖烷-5E,12E-二烯
YPTZ-12:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3,7-二(苯甲酰基)-1,13,14-三(羥基)-假白欖烷-5E,11E-二烯
YPTZ-13:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3-苯甲酰基-1,7,13,14-四(羥基)-假白欖烷-5E,11E-二烯
實施例9:YPTZ-14:(1S,2R,3S,4S,7R,9R,13R,14R,15S)-1,3,7,9,13,14,15-七(羥基)-假白欖烷-5E,11E-二烯,YPTZ-15:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-3-苯甲酰基-1,7,9,13,14,15-六(羥基)-假白欖烷-5E,11E-二烯,YPTZ-16:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-3,7-二(苯甲酰基)-1,9,13,14,15-五(羥基)-假白欖烷-5E,11E-二烯的制備
取(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3,7-二(苯甲酰基)-1,13,14-三(羥基)-假白欖烷-5E,11E-二烯(50mg)(以下簡記為YPTZ-12),加入1%NaOH甲醇溶液1mL,在室溫下攪拌1h,加入5mL水淬滅,乙酸乙酯萃取(3×5mL),合并有機層,干燥后減壓濃縮,通過半制備高效液相(CH3OH/H2O,7.5:2.5,3mL/min)純化得到YPTZ-14(8.1mg,tR10min),YPTZ-15(13.5mg,tR11min),和YPTZ-16(5.5mg,tR12min)。結構及數據如下:
YPTZ-14:[α]25D-10.5(c 0.17,MeOH);UV(MeOH)λmax(logε)210(3.51)nm;IR(KBr)νmax3419,2965,2930,1657,1081,1027,870cm-1;ESIMS m/z 771.4[2M-H]-;HREIMS m/z 421.2003[M+Cl]-(calcd for C20H34O7Cl,421.1999)。
1H NMR(CD3OD,400MHz)δH5.57(1H,d,J=10.6Hz,H-5),5.55(1H,d,J=15.7Hz,H-12),5.44(1H,d,J=15.7Hz,H-11),4.28(1H,d,J=6.5Hz,H-7),3.82(1H,d,J=11.7Hz,H-1),3.74(1H,dd,J=4.9,4.7Hz,H-3),3.64(1H,s,H-14),3.54(1H,dd,J=10.6,4.9Hz,H-4),3.47(1H,dd,J=3.4,3.4Hz,H-9),2.07(1H,dd,J=15.3,3.4Hz,H-8a),1.94(1H,m,H-2),1.77(1H,m,H-8b),1.71(3H,s,H3-17),1.28(3H,s,H3-20),1.08(3H,s,H3-18),1.06(3H,d,J=6.8Hz,H3-16),0.89(3H,s,H3-19);13C NMR(CD3OD,100MHz)δC138.9(C-6),135.9(C-11),131.0(C-12),121.2(C-5),89.8(C-1),84.2(C-15),79.7(C-14),77.1(C-3),75.7(C-13),73.9(C-7),72.9(C-9),45.7(C-2),43.8(C-4),41.0(C-10),38.0(C-8),30.6(C-20),24.1(C-19),20.1(C-18),17.1(C-17),11.9(C-16)。
YPTZ-15:[α]25D-56.0(c 0.26,MeOH);UV(MeOH)λmax(logε)272(2.98),229(4.07)nm;IR(KBr)νmax3470,2965,2932,1706,1452,1287,1085,1032cm-1;ESIMS m/z 525.2[M+Cl]-;HREIMS m/z 525.2271[M+Cl]-(calcd for C27H38O8Cl,525.2261)。
1H NMR(CD3OD,400MHz)δH5.62(1H,d,J=15.7Hz,H-12),5.58(1H,d,J=10.0Hz,H-5),5.52(1H,d,J=15.7Hz,H-11),5.38(1H,dd,J=4.3,4.3Hz,H-3),4.15(1H,d,J=6.4Hz,H-7),3.90(1H,d,J=11.9Hz,H-1),3.89(1H,dd,J=10.0,4.3Hz,H-4),3.76(1H,s,H-14),3.38(1H,dd,J=3.2,3.0Hz,H-9),2.27(1H,m,H-2),2.06(1H,dd,J=15.2,3.2Hz,H-8a),1.74(3H,s,H3-17),1.70(1H,m,H-8b),1.33(3H,s,H3-20),1.11(3H,s,H3-19),0.98(3H,d,J=6.7Hz,H3-16),0.90(3H,s,H3-18).3-OBz:8.03(2H,dd,J=7.2,1.5Hz),7.58(1H,ddd,J=7.5,7.2,1.5Hz),7.47(2H,dd,J=7.5,7.2Hz);13C NMR(CD3OD,100MHz)δC140.7(C-6),136.6(C-11),130.8(C-12),119.5(C-5),89.5(C-1),84.0(C-15),80.3(C-3),79.6(C-14),75.7(C-13),73.3(C-7),73.0(C-9),45.0(C-2),42.1(C-4),41.0(C-10),38.0(C-8),30.4(C-20),24.0(C-19),19.9(C-18),17.0(C-17),12.2(C-16).3-OBz:168.0,134.0,131.8,130.6×2,129.4×2。
YPTZ-16:[α]25D-45.0(c 0.20,CHCl3);UV(MeOH)λmax(logε)273(3.25),230(4.12)nm;IR(KBr)νmax3499,2963,2928,1719,1453,1282,1120,1068cm-1;ESIMS m/z 629.2[M+Cl]-;HREIMS m/z 629.2532[M+Cl]-(calcd for C34H42O9Cl,629.2523)。
1H NMR(CDCl3,400MHz)δH5.64(1H,d,J=15.7Hz,H-12),5.58(1H,d,J=15.7Hz,H-11),5.49(1H,d,J=10.2Hz,H-5),5.45(1H,dd,J=5.4,4.0Hz,H-3),5.31(1H,d,J=6.3Hz,H-7),4.01(1H,d,J=11.6Hz,H-1),3.83(1H,s,H-14),3.77(1H,dd,J=10.2,5.4Hz,H-4),3.66(1H,d,J=5.9Hz,HO-14),3.50(1H,s,H-9),2.51(1H,s,HO-13),2.17(1H,m,H-2),2.13(1H,m,H-8a),2.08(1H,s,HO-1),2.00(1H,m,H-8b),1.86(3H,s,H3-17),1.39(3H,s,H3-20),1.14(3H,s,H3-18),0.96(3H,d,J=6.7Hz,H3-16),0.93(3H,s,H3-19).3-OBz:7.77(2H,dd,J=7.5,1.0Hz),7.30(1H,ddd,J=7.5,7.3,1.0Hz),7.11(2H,dd,J=7.5,7.3Hz).7-OBz:7.54(2H,dd,J=7.1,1.0Hz),7.28(1H,ddd,J=7.5,7.1,1.0Hz),6.98(2H,dd,J=7.5,7.1Hz);13C NMR(CDCl3,100MHz)δC136.7(C-11),136.3(C-6),128.0(C-12),118.5(C-5),88.8(C-1),82.9(C-15),78.2(C-14),77.7(C-3),75.0(C-13),74.6(C-7),73.4(C-9),44.1(C-2),41.0(C-4),40.2(C-10),35.1(C-8),30.6(C-20),23.1(C-19),19.0(C-18),16.6(C-17),11.7(C-16).3-OBz:165.4,132.6,129.9,129.3×2,128.1×2.7-OBz:165.0,132.3,129.7,129.0×2,128.0×2。
實施例10
YPTZ-17:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-1,9,15-三(乙酰基)-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯,YPTZ-18:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-1,9,15-三(乙酰基)-3,7-二(苯甲酰基)-1,13-二(羥基)-假白欖烷-5E,11E-二烯的制備
取YPTZ-12(15mg),溶于2mL無水吡啶,攪拌下加入200μL乙酸酐,在室溫下攪拌12h,反應結束后加入1mL水淬滅,減壓濃縮,通過正相硅膠柱純化(洗脫劑為CH2Cl2)得到YPTZ-17(8.1mg),YPTZ-18(3.5mg)。結構及數據如下:
YPTZ-17:[α]25D-27.8(c 0.18,CHCl3);UV(MeOH)λmax(logε)274(3.11),228(4.13),204(3.92)nm;IR(KBr)νmax3518,2972,2933,1730,1453,1371,1280,1248,713cm-1;ESIMS m/z 755.1[M+Cl]-;HREIMS m/z 755.2839[M+Cl]-(calcd for C40H48O12Cl,755.2840)。
1H NMR(CDCl3,400MHz)δH5.82(1H,d,J=9.8Hz,H-5),5.60(1H,d,J=12.0Hz,H-1),5.52(1H,d,J=15.9Hz,H-12),5.45(1H,dd,J=5.4,4.0Hz,H-3),5.28(1H,d,J=15.9Hz,H-11),5.25(1H,d,J=6.1Hz,H-7),5.13(1H,dd,J=3.5,3.2Hz,H-9),4.72(1H,d,J=5.8Hz,H-14),4.05(1H,dd,J=9.8,4.6Hz,H-4),2.36(1H,m,H-2),2.05(2H,m,H2-8),1.82(3H,s,H3-17),1.33(3H,s,H3-20),0.86(3H,s,H3-18),0.86(3H,d,J=6.7Hz,H3-16),0.85(3H,s,H3-19).3-OBz:7.69(2H,dd,J=8.2,1.2Hz),7.27(1H,ddd,J=8.2,7.6,1.2Hz),7.07(2H,dd,J=8.2,7.6Hz).7-OBz:7.55(2H,dd,J=8.4,1.2Hz),7.24(1H,ddd,J=8.4,7.6,1.2Hz),6.97(2H,dd,J=8.4,7.6Hz).15-OAc:2.35(3H,s).1-OAc:2.20(3H,s).9-OAc:1.67(3H,s);13C NMR(CDCl3,100MHz)δC134.3(C-6),132.0(C-11),129.7(C-12),119.1(C-5),89.8(C-15),86.5(C-1),77.1(C-3),74.5(C-13),74.2(C-7),73.9(C-9),71.8(C-14),43.8(C-2),41.6(C-4),39.5(C-10),32.3(C-8),31.4(C-20),22.8(C-19),20.5(C-18),16.4(C-17),11.2(C-16).3-OBz:165.2,132.6,129.4,129.0×2,128.1×2.7-OBz:165.0,132.3,129.2,129.2×2,127.7×2.15-OAc:170.9,22.1.9-OAc:169.9,20.5.1-OAc:169.2,21.2。
YPTZ-18:[α]25D-41.0(c 0.06,CHCl3);UV(MeOH)λmax(logε)274(3.42),229(4.31)nm;IR(KBr)νmax3528,2959,2919,1730,1456,1374,1280,1113,1027,709cm-1;ESIMS m/z 743.3[M+Na]+;HREIMS m/z 743.3062[M+Na]+(calcd for C40H48O12Na,743.3038)。
1H NMR(CDCl3,400MHz)δH5.79(1H,d,J=9.6Hz,H-5),5.67(1H,s,H-14),5.55(1H,d,J=15.5Hz,H-12),5.47(1H,dd,J=4.5,4.2Hz,H-3),5.35(1H,d,J=15.5Hz,H-11),5.28(1H,d,J=3.2Hz,H-7),5.16(1H,dd,J=3.2,3.0Hz,H-9),4.25(1H,dd,J=9.5,4.5Hz,H-4),4.11(1H,dd,J=12.0,2.4Hz,H-1),3.79(1H,d,J=2.4Hz,HO-1),2.25(1H,m,H-2),2.07(2H,m,H2-8),1.85(3H,s,H3-17),1.20(1H,s,H-20),0.97(3H,s,H3-19),0.96(3H,s,H3-18),0.94(3H,d,J=6.7Hz,H3-16).3-OBz:7.65(2H,dd,J=7.5,1.4Hz),7.28(1H,ddd,J=8.0,7.2,1.2Hz),7.05(2H,dd,J=8.0,7.2Hz).7-OBz:7.55(2H,dd,J=7.3,1.1Hz),7.24(1H,ddd,J=8.0,7.3,1.1Hz),7.02(2H,dd,J=8.0,7.3Hz).15-OAc:2.45(3H,s).9-OAc:2.18(3H,s).14-OAc:1.70(3H,s);13C NMR(CDCl3,100MHz)δC134.1(C-6),132.5(C-11),129.2(C-12),119.2(C-5),90.8(C-15),86.6(C-1),77.0(C-3),75.4(C-13),74.2(C-7),73.8(C-9),73.0(C-14),42.6(C-2),42.4(C-4),39.7(C-10),32.4(C-8),31.5(C-20),22.8(C-19),20.6(C-18),16.4(C-17),11.6(C-16).3-OBz:165.2,132.6,129.5,128.9×2,128.1×2.7-OBz:165.0,132.2,129.4,129.2×2,127.8×2.15-OAc:173.1,22.2.14-OAc:170.1,20.9.9-OAc 170.0,21.6。
實施例11:YPTZ-19:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1-對甲基苯磺酰基-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-20:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1-對溴苯甲酰基-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-21:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1-呋喃酰基-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯;YPTZ-22:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1-噻吩酰基-3,7-二(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯及YPTZ-23:(1S,2R,3S,4S,7R,9R,13R,14R,15R)-9,15-二(乙酰基)-1,3,7-三(苯甲酰基)-13,14-二(羥基)-假白欖烷-5E,11E-二烯的制備
取YPTZ-12(5組,各15mg),溶于2mL無水吡啶,攪拌下分別加入200μL對甲苯磺酰氯、對溴苯甲酰氯、2-呋喃甲酰氯、2-噻吩甲酰氯、苯甲酰氯,在室溫下攪拌12h,反應結束后加入1mL水淬滅,減壓濃縮,通過正相硅膠柱純化(洗脫劑為CH2Cl2)得到YPTZ-19(6mg),YPTZ-20(8mg),YPTZ-21(7.5mg),YPTZ-22(7mg),YPTZ-23(8mg)。結構及數據如下:
YPTZ-19:[α]25D-20.6(c 0.05,CHCl3);UV(MeOH)λmax(logε)273(3.33),227(4.36),208(4.15)nm;IR(KBr)νmax3448,2965,2927,1723,1454,1369,1280,1180,1116,711cm-1;ESIMS m/z 855.1[M+Na]+;HREIMS m/z 855.3057[M+Na]+(calcd for C45H52O13SNa,855.3021)。
1H NMR(CDCl3,400MHz)δH6.21(1H,d,J=11.6Hz,H-1),5.66(1H,d,J=15.7Hz,H-12),5.49(1H,d,J=9.3Hz,H-5),5.48(1H,dd,J=6.5,3.5Hz,H-3),5.42(1H,d,J=15.7Hz,H-11),5.21(1H,dd,J=3.6,3.5Hz,H-7),4.95(1H,dd,J=3.5,3.3Hz,H-9),4.23(1H,dd,J=9.3,6.5Hz,H-4),4.17(1H,s,H-14),2.55(1H,m,H-2),2.04(2H,m,H2-8),1.78(3H,s,H3-17),1.38(1H,s,H-20),0.99(3H,s,H3-19),0.95(3H,s,H3-18),0.40(3H,d,J=6.8Hz,H3-16).3-OBz:7.92(2H,dd,J=7.3,1.0Hz),7.38(1H,ddd,J=7.7,7.3,1.0Hz),7.19(2H,dd,J=7.7,7.3Hz),7-OBz:7.59(2H,dd,J=7.3,1.0Hz),7.20(1H,ddd,J=7.7,7.3,1.0Hz),7.02(2H,dd,J=7.7,7.3Hz),1-O-tosyl:7.83(2H,d,J=8.3,Hz),7.31(2H,d,J=8.3Hz),2.38(3H,s).15-OAc:2.47(3H,s).9-OAc:1.72(3H,s);13C NMR(CDCl3,100MHz)δC135.7(C-6),131.0(C-12),130.5(C-11),118.6(C-5),90.3(C-15),88.4(C-1),75.8(C-14),75.3(C-13),74.6(C-3),73.9(C-9),73.7(C-7),41.1(C-2),40.3(C-4),39.7(C-10),32.4(C-8),31.8(C-20),23.3(C-19),20.9(C-18),16.0(C-17),10.7(C-16).3-OBz:165.9,132.4,129.6×2,129.5,127.8×2.7-OBz:164.8,132.2,129.5×2,129.4,127.8×2.1-O-tosyl:145.4,132.9,129.8×2,128.3×2,21.7.15-OAc:170.7,22.8.9-OAc:169.7,20.9。
YPTZ-20:[α]25D-51.9(c 0.12,CHCl3);UV(MeOH)λmax(logε)239(4.49),nm;IR(KBr)νmax3565,2974,1729,1588,1278,1098,710cm-1;ESIMS m/z 883.1[M+Na]+;HREIMS m/z 883.2329[M+Na]+(calcd for C45H49BrO12Na,883.2300)。
1H NMR(CDCl3,400MHz)δH5.87(1H,d,J=9.8Hz,H-5),5.83(1H,d J=11.7Hz,H-1),5.52(1H,dd,J=5.3,4.3Hz,H-3),5.51(1H,d,J=15.8Hz,H-11),5.30(1H,d,J=15.8Hz,H-12),5.29(1H,d,J=3.8Hz,H-7),5.14(1H,dd,J=3.5,2.9Hz,H-9),4.81(1H,d,J=5.8Hz,H-14),4.09(1H,dd,J=9.8,5.3Hz,H-4),2.53(1H,m,H-2),2.07(2H,m,H2-8),1.86(3H,s,H3-17),1.38(1H,s,H-20),0.94(3H,s,H3-19),0.94(3H,s,H3-18),0.93(3H,d,J=6.7Hz,H3-16).1-O-bromobenzoyl:7.89(2H,d,J=8.5Hz),7.64(2H,d,J=8.5Hz).3-OBz:7.74(2H,dd,J=7.3,1.5Hz),7.30(1H,ddd,J=7.7,7.3,1.5Hz),7.12(2H,dd,J=7.7,7.3Hz).7-OBz:7.57(2H,dd,J=7.3,1.4Hz),7.28(1H,ddd,J=7.7,7.3,1.4Hz),6.99(2H,dd,J=7.7,7.3Hz).15-OAc:2.37(3H,s).9-OAc:1.68(3H,s);13C NMR(CDCl3,100MHz)δC134.5(C-6),132.1(C-11),129.9(C-12),119.2(C-5),90.1(C-15),87.3(C-1),77.1(C-3),74.8(C-13),74.2(C-7),73.8(C-9),72.2(C-14),44.0(C-2),41.8(C-4),39.6(C-10),32.3(C-8),31.5(C-20),22.8(C-19),20.6(C-18),16.5(C-17),11.4(C-16).1-O-bromobenzoyl:164.9,132.1×2,130.9×2,129.5,128.4.3-OBz:165.3,132.7,129.5,129.0×2,128.2×2.7-OBz:165.0,132.3,129.2×2,129.0,127.8×2.15-OAc:171.0,22.1.9-OAc:170.0,20.9。
YPTZ-21:[α]25D-16.0(c 0.30,CHCl3);UV(MeOH)λmax(logε)230(4.38),208(4.17)nm;IR(KBr)νmax3511,2970,2933,1730,1583,1280,1113,710cm-1;ESIMS m/z 795.3[M+Na]+;HREIMS m/z 795.3011[M+Na]+(calcd for C43H48O13Na,795.2987)。
1H NMR(CDCl3,400MHz)δH5.88(1H,d,J=9.8Hz,H-5),5.75(1H,d J=11.5Hz,H-1),5.57(1H,d,J=11.5Hz,H-11),5.52(1H,dd,J=5.0,4.3Hz,H-3),5.32(1H,d,J=15.5Hz,H-12),5.31(1H,d,J=3.5Hz,H-7),5.17(1H,dd,J=3.3,3.1Hz,H-9),4.81(1H,d,J=5.9Hz,H-14),4.13(1H,dd,J=9.8,5.0Hz,H-4),2.52(1H,m,H-2),2.09(2H,dd,J=3.5,3.3,H2-8),1.87(3H,s,H3-17),1.37(1H,s,H-20),0.95(3H,s,H3-19),0.94(3H,s,H3-18),0.94(3H,d,J=6.8Hz,H3-16).3-OBz:7.75(2H,dd,J=8.3,1.2Hz),7.29(1H,ddd,J=8.3,7.8,1.2Hz),7.13(2H,dd,J=8.3,7.8Hz).7-OBz:7.60(2H,dd,J=8.2,1.3Hz),7.27(1H,ddd,J=8.2,7.6,1.3Hz),6.99(2H,dd,J=8.2,7.6Hz).1-O-2-furoyl:7.59(1H,dd,J=3.5,1.3Hz),7.33(1H,dd,J=3.5,0.7Hz),6.56(1H,dd,J=3.5,1.7Hz).15-OAc:2.39(3H,s).9-OAc:1.68(3H,s);13C NMR(CDCl3,100MHz)δC134.3(C-6),132.0(C-11),129.7(C-12),119.1(C-5),90.0(C-15),86.9(C-1),77.4(C-3),74.5(C-13),74.2(C-7),73.8(C-9),72.0(C-14),44.0(C-2),41.8(C-4),39.5(C-10),32.2(C-8),31.4(C-20),22.7(C-19),20.5(C-18),16.4(C-17),11.2(C-16).1-O-2-furoyl:156.8,146.2,144.3,118.8,112.2.3-OBz:165.1,132.6,129.4,129.0×2,128.1×2.7-OBz:165.0,132.1,129.2,129.1×2,127.7×2.15-OAc:170.8,22.0.9-OAc:169.9,20.7。
YPTZ-22:[α]25D-12.8(c 0.41,CHCl3);UV(MeOH)λmax(logε)274(4.01),233(4.30)208(4.08)nm;IR(KBr)νmax3479,2971,2933,1730,1453,1280,1096,710cm-1;ESIMS m/z 811.3[M+Na]+;HREIMS m/z 811.2783[M+Na]+(calcd for C43H48O12SNa,811.2759)。
1H NMR(CDCl3,400MHz)δH5.86(1H,d,J=9.8Hz,H-5),5.73(1H,d J=11.6Hz,H-1),5.55(1H,d,J=15.6Hz,H-11),5.51(1H,dd,J=5.0,4.5Hz,H-3),5.30(1H,d,J=15.6Hz,H-12),5.29(1H,d,J=3.0Hz,H-7),5.14(1H,dd,J=3.1,3.1Hz,H-9),4.84(1H,d,J=5.8Hz,H-14),4.10(1H,dd,J=9.8,5.0Hz,H-4),3.43(1H,d,J=5.8Hz,HO-14),2.83(1H,s,HO-13),2.49(1H,m,H-2),2.07(2H,dd,J=3.5,3.1Hz,H2-8),1.86(3H,s,H3-17),1.38(1H,s,H-20),0.96(3H,d,J=6.8Hz,H3-16),0.94(3H,s,H3-18),0.93(3H,s,H3-19).1-O-thiopheene-2-carbonyl:7.89(1H,d,J=3.7Hz),7.60(1H,d,J=4.9Hz),7.17(1H,dd,J=4.9,3.7Hz).3-OBz:7.73(2H,dd,J=7.3,1.0Hz),7.31(1H,ddd,J=7.7,7.3,1.0Hz),7.12(2H,dd,J=7.7,7.3Hz).7-OBz:7.57(2H,dd,J=7.3,1.2Hz),7.26(1H,ddd,J=7.8,7.3,1.2Hz),6.98(2H,dd,J=7.8,7.3Hz).15-OAc:2.38(3H,s).9-OAc:1.67(3H,s);13C NMR(CDCl3,100MHz)δC134.5(C-6),132.1(C-11),129.6(C-12),119.1(C-5),90.1(C-15),87.4(C-1),77.4(C-3),74.6(C-13),74.2(C-7),73.9(C-9),72.1(C-14),44.1(C-2),41.8(C-4),39.6(C-10),32.3(C-8),31.5(C-20),22.8(C-19),20.6(C-18),16.5(C-17),11.4(C-16).1-O-thiopheene-2-carbonyl:160.6,134.1,132.9,132.2,128.4.3-OBz:165.2,132.7,129.5,129.1×2,128.2×2.7-OBz:165.0,132.2,129.3,129.2×2,127.8×2.15-OAc:171.0,22.1.9-OAc:170.0,20.8。
YPTZ-23:[α]25D-13.2(c 0.50,CHCl3);UV(MeOH)λmax(logε)274(3.50),231(4.43)nm;IR(KBr)νmax3500,2971,2935,1730,1452,1280,1113,1027,982,710cm-1;ESIMS m/z 805.3[M+Na]+;HREIMS m/z 805.3217[M+Na]+(calcd for C45H50O12Na,805.3194)。
1H NMR(CDCl3,400MHz)δH5.89(1H,d,J=9.7Hz,H-5),5.88(1H,d J=11.8,H-1),5.54(1H,dd,J=5.3,4.3Hz,H-3),5.54(1H,d,J=15.5Hz,H-11),5.33(1H,d,J=15.5Hz,H-12),5.30(1H,s,Hz,H-7),5.15(1H,dd,J=3.5,2.9Hz,H-9),4.86(1H,d,J=5.6Hz,H-14),4.11(1H,dd,J=9.7,5.1Hz,H-4),2.52(1H,m,H-2),2.08(2H,m,H2-8),1.87(3H,s,H3-17),1.39(1H,s,H-20),0.95(3H,s,H3-19),0.94(3H,s,H3-18),0.93(3H,d,J=6.7Hz,H3-16).1-OBz:8.02(2H,dd,J=7.3,1.2Hz),7.61(1H,ddd,J=7.7,7.3,1.2Hz),7.50(2H,dd,J=7.7,7.3Hz).3-OBz:7.60(2H,dd,J=7.3,1.1Hz),7.31(1H,ddd,J=7.8,7.3,1.1Hz),7.13(2H,dd,J=7.8,7.3Hz).7-OBz:7.58(2H,dd,J=7.2,1.0Hz),7.28(1H,ddd,J=7.8,7.2,1.0Hz),7.00(2H,dd,J=7.8,7.2Hz).15-OAc:2.38(3H,s).9-OAc:1.67(3H,s);13C NMR(CDCl3,100MHz)δC134.4(C-6),132.1(C-11),129.9(C-12),119.3(C-5),90.1(C-15),87.1(C-1),77.2(C-3),74.7(C-13),74.2(C-7),73.9(C-9),72.3(C-14),44.2(C-2),41.9(C-4),39.6(C-10),32.4(C-8),31.5(C-20),22.8(C-19),20.6(C-18),16.5(C-17),11.4(C-16).1-OBz:165.4,133.2,130.1,129.3×2,128.7×2.3-OBz:165.3,132.7,129.5,129.1×2,128.2×2.7-OBz:165.0,132.2,129.3,129.3×2,127.8×2.15-OAc:170.9,22.1.9-OAc:170.0,20.8。
實施例12:YPTZ-24:(4S,9R,13R,14R,15R)-9,15-二(乙酰基)-13,14-二(羥基)-假白欖烷-2Z,5E,11E-三烯的制備
取YPTZ-13(15mg),溶于無水二氯甲烷(5mL),加入m-CPBA(15mg),在室溫下攪拌3h。減壓濃縮后通過正相硅膠柱(洗脫劑為CH2Cl2)純化得到YPTZ-31(10mg),結構及數據如下:
YPTZ-31:[α]25D62.7(c 0.26,CHCl3);UV(MeOH)λmax(logε)231(3.93),205(3.76)nm;IR(KBr)νmax2963,2913,1752,1727,1668,1261,1098,1020,800cm-1;ESIMS m/z 737.3[M+Na]+;HREIMS m/z 737.2809[M+Na]+(calcd for C37H46O14Na,737.2780)。
1H NMR(CDCl3,400MHz)δH7.20(1H,dd,J=9.4,1.3Hz,H-5),5.98(1H,s,H-14),5.84(1H,d,J=8.6Hz,H-9),5.66(1H,dd,J=4.0,3.7Hz,H-3),5.49(1H,d,J=10.7Hz,H-1),5.36(1H,d,J=4.0Hz,H-8),3.79(1H,dd,J=9.4,4.0Hz,H-4),3.73(1H,dd,J=5.5,2.0Hz,H-12),2.25(1H,m,H-2),1.92(3H,s,H-17),1.47(1H,d,J=16.8Hz,Ha-11),1.11(1H,d,J=5.5Hz,Hb-11),1.06(3H,s,H3-18),1.05(3H,d,J=6.8Hz,H3-16)1.00(3H,s,H3-19).3-OBz:8.07(2H,dd,J=8.3,1.3Hz),7.60(1H,ddd,J=8.3,7.8,1.3Hz),7.51(1H,dd,J=8.3,7.8Hz).15-OAc:2.37(3H,s).14-OAc:2.21(3H,s).9-OAc:2.08(3H,s).1-OAc:2.03(3H,s).8-OAc:1.20(3H,s);13C NMR(CDCl3,100MHz)δC194.1(C-7),138.4(C-6),138.4(C-5),89.7(C-15),85.2(C-1),80.5(C-8),77.3(C-3),76.5(C-9),69.2(C-14),59.8(C-13),56.2(C-12),46.9(C-4),45.8(C-2),37.2(C-11),37.1(C-10),25.8(C-19),22.4(C-18),16.3(C-20),13.2(C-17),11.9(C-16).3-OBz:165.8,133.6,129.9×2,129.4,128.8×2.9-OAc:169.8,21.1.1-OAc:169.8,20.6.8-OAc:169.5,19.3.15-OAc:169.2,22.4.14-OAc:168.9,21.8。
實施例13:YPTZ-25:(1S,2R,3S,4S,7R,9R,13R,15S)-1,9,15-三(乙酰基)-3,7-二(苯甲酰基)-13-羥基-14羰基-假白欖烷-5E,11E-二烯的制備
取YPTZ-17(15mg),溶于5mL無水二氯甲烷,加入戴斯-馬丁試劑(15mg),在室溫下攪拌3h,過濾,濾液減壓濃縮,通過半制備高效液相(CH3OH/H2O,7.5:2.5,3mL/min)純化得到YPTZ-25(4.5mg,tR13min)。結構及數據如下:
YPTZ-25:[α]25D-41.3(c 0.14,CHCl3);UV(MeOH)λmax(logε)273(3.45),232(4.27)nm;IR(KBr)νmax3533,2961,2917,1753,1729,1455,1375,1278,1263,1071,803,712cm-1;ESIMS m/z 741.3[M+Na]+;HREIMS m/z 741.2902[M+Na]+(calcd for C40H46O12Na,741.2881)。
1H NMR(CDCl3,400MHz)δH5.84(1H,d,J=10.5Hz,H-5),5.72(1H,d,J=15.6Hz,H-11),5.62(1H,d,J=8.0Hz,H-1),5.59(1H,d,J=15.6Hz,H-12),5.50(1H,dd,J=3.8,3.8Hz,H-3),5.35(1H,d,J=6.1Hz,H-7),5.10(1H,d,J=4.4Hz,H-9),3.84(1H,s,HO-13),3.69(1H,dd,J=10.5,4.0Hz,H-4),2.60(1H,m,H-2),2.18(1H,m,H-8a),1.86(1H,d,J=15.6Hz,H-8b),1.59(3H,s,H3-17),1.41(3H,s,H3-20),1.03(3H,d,J=6.8Hz,H3-16),0.98(3H,s,H3-18),0.98(3H,s,H3-19).3-OBz:7.92(2H,dd,J=7.4,1.2Hz),7.62(1H,ddd,J=7.5,7.4,1.2Hz),7.45(2H,dd,J=7.5,7.4Hz).7-OBz:7.66(2H,dd,J=7.2,1.0Hz),7.29(1H,ddd,J=7.5,7.2,1.0Hz),6.91(2H,dd,J=7.5,7.2Hz).15-OAc:2.51(3H,s).1-OAc:2.08(3H,s).9-OAc:1.47(3H,s);13C NMR(CDCl3,100MHz)δC213.1(C-14),136.9(C-11),136.6(C-6),116.8(C-5),95.3(C-15),84.7(C-1),79.7(C-13),78.1(C-3),74.3(C-9),73.8(C-7),54.3(C-4),45.4(C-2),39.7(C-10),31.5(C-8),27.8(C-20),23.8(C-19),20.9(C-18),16.0(C-17),11.6(C-16).3-OBz:165.5,133.1,129.54×2,129.51,128.6×2.7-OBz:164.9,132.6,129.4×2,129.3,127.9×2,15-OAc:170.9,22.0.9-OAc:170.2,20.6.1-OAc:169.6,21.0。
實施例14:YPTZ-26:(1S,2S,3S,4S,7R,9R,13R,14R,15S)-9,15-二(乙酰基)-3,7-二(苯甲酰基)-1,13,14-三(羥基)-假白欖烷-5E,11E-烯的制備
取YPTZ-12(15mg),溶于5mL無水二氯甲烷,加入10%Pd/C,通入氫氣,在室溫下攪拌10h。反應液過濾,濾液減壓濃縮,通過正相硅膠柱(洗脫劑為CH2Cl2/MeOH,300:1)純化得到YPTZ-26(9mg)。結構及數據如下:
YPTZ-26:[α]25D28.0(c 0.17,CHCl3);UV(MeOH)λmax(logε)273(3.34),232(4.24)nm;IR(KBr)νmax3468,2961,2931,1727,1713,1265,1025,711cm-1;ESIMS m/z 679.0[M-H]-;HREIMS m/z 703.3110[M+Na]+(calcd for C38H48O11Na,703.3089).
1H NMR(CDCl3,400MHz)δH6.15(1H,d,J=9.8Hz,H-5),5.66(1H,d,J=6.5Hz,H-9),5.47(1H,dd,J=4.6,4.0Hz,H-3),5.37(1H,d,J=6.4Hz,H-7),4.73(1H,d,J=5.1Hz,HO-13),4.52(1H,dd,J=3.7Hz,HO-14),4.30(1H,dd,J=11.7,3.4Hz,H-1),4.24(1H,d,J=3.7Hz,H-14),4.20(1H,d,J=3.5Hz,HO-1),4.07(1H,dd,J=9.8,4.6Hz,H-4),2.50(1H,dd,J=12.8,5.2Hz,H-12b),2.25(1H,m,H-2),2.15(1H,m,H-8a),1.91(1H,m,H-8b),1.83(3H,s,H3-17),1.57(1H,d,J=12.8Hz,H-11a),1.23(3H,s,H3-20),1.14(1H,ddd,J=5.6,5.5,5.2Hz,H-11b),1.02(3H,d,J=6.6Hz,H3-16),0.84(3H,s,H3-18),0.75(3H,s,H3-19).3-OBz:7.79(2H,dd,J=8.3,1.2Hz),7.43(1H,ddd,J=8.3,7.6,1.2Hz),7.24(2H,dd,J=8.3,7.6Hz).7-OBz:7.61(2H,dd,J=8.3,1.2Hz),7.29(1H,ddd,J=8.3,7.6,1.2Hz),6.96(2H,dd,J=8.3,7.6Hz).15-OAc:2.42(3H,s).9-OAc:1.41(3H,s);13C NMR(CDCl3,100MHz)δC137.9(C-6),118.5(C-5),91.3(C-15),87.8(C-1),78.1(C-3),74.7(C-7),73.4(C-13),72.8(C-14),72.0(C-9),43.5(C-2),41.9(C-4),37.4(C-10),32.0(C-11),31.5(C-8),29.1(C-12),27.2(C-20),24.1(C-19),23.0(C-18),17.2(C-17),11.8(C-16).3-OBz:165.2,132.7,129.8,129.4×2,128.3×2.7-OBz:165.2,132.3,129.6,129.1×2,127.8×2.15-OAc:173.1,22.0.9-OAc:170.4,20.7。
實施例15:YPTZ-27:(1S,2R,8R,9S,14R,15S)-9,15-二(乙酰基)-1,8,14-trihydroxy-7-羰基-假白欖烷-3Z,5E,12E-三烯,YPTZ-28:(1S,2R,8R,9S,14R,15S)-1,9,14,15-四(乙酰基)-8-羥基-7-羰基-假白欖烷-3Z,5E,12E-三烯的制備
取YPTZ-1(20mg),加入1%NaOH甲醇溶液1mL,在室溫下攪拌1h,加入5mL水淬滅,乙酸乙酯萃取(3×5mL),合并有機層,干燥后減壓濃縮,通過半制備高效液相(CH3OH/H2O,7.5:2.5,3mL/min)純化得到YPTZ-27(4.1mg,tR12min)和YPTZ-28(3.5mg,tR13min)。結構及數據如下:
YPTZ-27:[α]25D-58.8(c 0.03,CDCl3);UV(MeOH)λmax(logε)255(3.80),209(4.02)nm;IR(KBr)νmax 3476,2959,2918,1736,1711,1676,1242,1107,808cm-1;EIMS m/z 473.1[M+Na]+;HREIMS m/z 451.1023[M+H]+(calcd for C24H35O8,451.2326)。
1H NMR(CDCl3,400MHz)δH6.89(1H,s,H-5),5.83(1H,s,H-3),5.38(1H,m,H-12),5.02(1H,s,H-9),4.93(1H,m,H-8),4.92(1H,s,H-14),4.71(1H,d,J=2.3Hz,HO-1),4.38(1H,d,J=2.2Hz,HO-14),4.12(1H,dd J=7.5,2.3Hz,H-1),3.56(1H,d,J=8.1Hz,HO-8),3.02,(1H,m,H-2),2.73(1H,dd,J=15.8,11.8Hz,H-11a),2.06(1H,m,H-11b),1.83(3H,s,H3-20),1.80(3H,s,H3-17),1.24(3H,d,J=7.0Hz,H3-16),1.20(3H,s,H3-18),0.98(3H,s,H3-19).15-OAc:2.17(3H,s).9-OAc:2.01(3H,s);13C NMR(CDCl3,100MHz)δC201.5(C-7),138.3(C-3),137.2(C-5),137.1(C-13),136.3(C-6),134.3(C-4),122.8(C-12),97.8(C-15),87.6(C-1),77.7(C-9),75.4(C-14),70.7(C-8),45.2(C-2),40.0(C-10),39.2(C-11),25.3(C-19),24.8(C-18)18.5(C-16),14.0(C-20),12.0(C-17).15-OAc:173.4,22.0.9-OAc:170.1,20.5。
YPTZ-28:[α]25D-68.6(c 0.07,CDCl3);UV(MeOH)λmax(logε)255(3.75),209(3.93)nm;IR(KBr)νmax 3469,2959,2922,1743,1676,1246,1217,1043,1024,882cm-1;EIMS m/z 557.1[M+Na]+;HREIMS m/z 557.2374[M+Na]+(calcd for C28H38O10Na,557.2357)。
1H NMR(CDCl3,400MHz)δH6.97(1H,s,H-5),5.84(1H,dd,J=1.8,1.7Hz,H-3),5.80(1H,m,H-12),5.80(1H,s,H-14),5.79(1H,d,J=7.0Hz,H-1),4.88(1H,s,H-8),4.87(1H,s,H-9),3.59(1H,d,J=8.0Hz,HO-8),2.98(1H,m,H-2),2.72(1H,dd,J=15.5,12.1Hz,H-11a),1.82(3H,s,H3-17),1.68(3H,s,H3-20),1.22(3H,d,J=7.1Hz,H3-16),1.19(3H,s,H3-18),0.99(3H,s,H3-19).14-OAc:2.15(3H,s).1-OAc:2.06(3H,s).15-OAc:2.04(3H,s).9-OAc:1.98(3H,s);13C NMR(CDCl3,100MHz)δC201.1(C-7),137.0(C-6),136.7(C-3),136.3(C-5),135.4(C-4),133.5(C-13),125.3(C-12),94.0(C-15),83.0(C-1),78.3(C-9),74.8(C-14),70.8(C-8),44.1(C-2),40.2(C-10),39.7(C-11),25.5(C-19),24.7(C-18),17.9(C-16),14.9(C-20),11.5(C-17).1-OAc:170.7,21.0.9-OAc:169.9,22.1.15-OAc:169.7,20.3.14-OAc:169.4,20.6。
實施例16:YPTZ-29:(1S,2R,3S,4S,5S,8R,9S,14R,15S)-7,8,9,14,15-五(乙酰基)-1,3-二(苯甲酰基)-5-羥基-假白欖烷-6E,12E-二烯的制備
取YPTZ-10,按實施例11的方法制備得到。結構及數據如下:
YPTZ-29:[α]25D-104.1(c 0.17,CHCl3);UV(MeOH)λmax(logε)273(3.32),232(4.24)nm;IR(KBr)νmax3483,2966,2926,1732,1373,1274,1229,1112,1027,714cm-1;ESIMS m/z 843.3[M+Na]+;HREIMS m/z 843.3225[M+Na]+(calcd for C44H52O15Na,843.3198)。
1H NMR(CDCl3,400MHz)δH6.58(1H,s,H-8),6.17(1H,s,H-14),5.99(1H,d,J=8.8Hz,H-12),5.88(1H,d,J=11.7Hz,H-1),5.55(1H,d,J=4.0,3.9Hz,H-3),5.28(1H,s,H-9),4.62(1H,dd,J=11.0,2.8Hz,H-5),4.43(1H,dd,J=11.0,4.6Hz,H-4),2.69(1H,m,H-2),2.39(1H,dd,J=16.4,9.5Hz,H-11a),2.00(1H,m,H-11b),1.65(1H,s,H-20),1.35(3H,s,H3-17),1.01(3H,s,H3-18),0.96(3H,d,J=6.8Hz,H3-16),0.90(3H,s,H3-19).1-OBz:8.04(2H,dd,J=7.2,1.0Hz),7.57(1H,ddd,J=7.5,7.2,1.0Hz),7.46(1H,dd,J=7.5,7.2Hz).3-OBz:8.01(2H,dd,J=8.2,1.0Hz),7.56(1H,ddd,J=8.2,7.5,1.1Hz),7.45(1H,dd,J=8.2,7.5Hz).14-OAc:2.26(3H,s).7-OAc:2.16(3H,s).8-OAc:2.14(3H,s).15-OAc:2.10(3H,s).9-OAc:2.04(3H,s);13C NMR(CDCl3,100MHz)δC143.8(C-7),124.8(C-12),91.4(C-15),84.5(C-1),78.5(C-9),75.9(C-3),72.8(C-14),71.4(C-5),69.5(C-8),47.4(C-4),44.2(C-2),41.2(C-11),38.7(C-10),29.3(C-19),20.5(C-18),17.7(C-17),17.3(C-20),11.9(C-16).1-OBz:166.4,133.0,130.1,129.8×2,128.4×2.3-OBz:165.4,133.2,129.9,129.5×2,128.6×2.8-OAc:170.3,21.2.9-OAc:170.3,21.8.14-OAc:169.2,21.0.7-OAc:169.1,20.1.15-OAc:167.8,20.9。
實施例17:YPTZ-30:(1S,2S,3S,4S,5S,8R,9S,14R,15S)-二(乙酰基)-3-苯甲酰基-1,5,9,14,15-五(羥基)-假白欖烷-6E,12E-二烯的制備
取YPTZ-9,按照實施例9的方法可制備題述化合物。結構及數據如下:
YPTZ-30:[α]25D-12.0(c 0.13,CHCl3);UV(MeOH)λmax(logε)273(3.04),229(4.12)211(3.97)nm;IR(KBr)νmax3409,2972,2920,1752,1726,1372,1260,1228,1083,1025,713cm-1;ESIMS m/z 655.3[M+Na]+;HREIMS m/z 655.2748[M+Na]+(calcd for C31H42O11Na,655.2619)。
1H NMR(CDCl3,400MHz)δH6.70(1H,s,H-8),5.85(1H,s,H-12),5.38(1H,d,J=3.7,3.6Hz,H-3),5.08(1H,s,H-9),4.52(1H,s,H-14),4.47(1H,d,J=11.1Hz,H-5),4.05(1H,d,J=9.3Hz,H-1),3.78(1H,dd,J=11.1,3.6Hz,H-4),2.40(1H,m,H-11a),2.23(1H,m H-2),1.94(1H,m,H-11b),1.76(1H,s,H-20),1.27(3H,s,H3-17),1.01(3H,s,H3-18),0.97(3H,d,J=6.5Hz,H3-16),0.86(3H,s,H3-19).1-OBz:7.98(2H,dd,J=7.5,1.0Hz),7.47(1H,ddd,J=7.5,7.2,1.0Hz),7.34(1H,dd,J=7.5,7.4Hz).9-OAc:2.10(3H,s).7-OAc:2.05(3H,s).8-OAc:2.01(3H,s);13C NMR(CDCl3,100MHz)δC143.1(C-7),136.9(C-13),121.7(C-12),88.8(C-1),85.1(C-15),78.9(C-9),77.7(C-14),76.0(C-3),71.0(C-5),69.9(C-8),45.4(C-2),44.6(C-4),41.2(C-11),38.7(C-10),29.3(C-19),20.7(C-18),18.0(C-17),18.9(C-20),11.9(C-16).3-OBz:165.5,132.9,129.9,129.5×2,128.4×2.8-OAc:170.9,21.0.9-OAc:170.9,21.0.7-OAc:167.8,20.1。
實施例18:YPTZ-31:(1S,2R,3S,4S,8R,9S,12S,13R,14R,15S)-1,8,9,14,15-五(乙酰基)-3-苯甲酰基-12,13-醚-7-羰基-5E-烯的制備
取YPTZ-11(15mg),溶于無水二氯甲烷(5mL),加入m-CPBA(15mg),在室溫下攪拌3h。減壓濃縮后通過正相硅膠柱(洗脫劑為CH2Cl2)純化得到YPTZ-31(10mg),結構及數據如下:
YPTZ-31:[α]25D62.7(c 0.26,CHCl3);UV(MeOH)λmax(logε)231(3.93),205(3.76)nm;IR(KBr)νmax2963,2913,1752,1727,1668,1261,1098,1020,800cm-1;ESIMS m/z 737.3[M+Na]+;HREIMS m/z 737.2809[M+Na]+(calcd for C37H46O14Na,737.2780)。
1H NMR(CDCl3,400MHz)δH7.20(1H,dd,J=9.4,1.3Hz,H-5),5.98(1H,s,H-14),5.84(1H,d,J=8.6Hz,H-9),5.66(1H,dd,J=4.0,3.7Hz,H-3),5.49(1H,d,J=10.7Hz,H-1),5.36(1H,d,J=4.0Hz,H-8),3.79(1H,dd,J=9.4,4.0Hz,H-4),3.73(1H,dd,J=5.5,2.0Hz,H-12),2.25(1H,m,H-2),1.92(3H,s,H-17),1.47(1H,d,J=16.8Hz,Ha-11),1.11(1H,d,J=5.5Hz,Hb-11),1.06(3H,s,H3-18),1.05(3H,d,J=6.8Hz,H3-16)1.00(3H,s,H3-19).3-OBz:8.07(2H,dd,J=8.3,1.3Hz),7.60(1H,ddd,J=8.3,7.8,1.3Hz),7.51(1H,dd,J=8.3,7.8Hz).15-OAc:2.37(3H,s).14-OAc:2.21(3H,s).9-OAc:2.08(3H,s).1-OAc:2.03(3H,s).8-OAc:1.20(3H,s);13C NMR(CDCl3,100MHz)δC194.1(C-7),138.4(C-6),138.4(C-5),89.7(C-15),85.2(C-1),80.5(C-8),77.3(C-3),76.5(C-9),69.2(C-14),59.8(C-13),56.2(C-12),46.9(C-4),45.8(C-2),37.2(C-11),37.1(C-10),25.8(C-19),22.4(C-18),16.3(C-20),13.2(C-17),11.9(C-16).3-OBz:165.8,133.6,129.9×2,129.4,128.8×2.9-OAc:169.8,21.1.1-OAc:169.8,20.6.8-OAc:169.5,19.3.15-OAc:169.2,22.4.14-OAc:168.9,21.8。
實施例19:YPTZ-32:(1S,2R,3S,4S,8R,9S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-5-羰基-假白欖烷--6E,12E-二烯的制備
取YPTZ-9(15mg),溶于5mL無水二氯甲烷,加入戴斯-馬丁試劑(15mg),在室溫下攪拌3h,過濾,濾液減壓濃縮,通過正相硅膠柱純化(洗脫劑為CH2Cl2/MeOH,200:1)得到YPTZ-32,結構及數據如下:
YPTZ-32:[α]25D-115.9(c 0.12,CHCl3);UV(MeOH)λmax(logε)273(2.90),230(3.98)206(3.86)nm;IR(KBr)νmax3646,3558,2974,1744,1374,1247,1226,1095,1058,718cm-1;ESIMS m/z 779.2[M+Na]+;HREIMS m/z 779.2917[M+Na]+(calcd for C39H48O15Na,779.2885)。
1H NMR(CDCl3,400MHz)δH6.20(1H,s,H-8),6.09(1H,s,H-14),6.00(1H,d,J=4.1,3.8Hz,H-3),5.71(1H,d,J=9.4Hz,H-12),5.48(1H,d,J=11.1Hz,H-1),4.82(1H,d,J=4.1Hz,H-4),4.75(1H,s,H-9),2.46(1H,m,H-2),2.26(1H,m,H-11a),1.97(H-11b),1.82(1H,s,H-20),1.33(3H,s,H3-17),0.97(3H,d,J=6.8Hz,H3-16),0.95(3H,s,H3-19),0.85(3H,s,H3-18).3-OBz:7.98(2H,dd,J=8.3,1.2Hz),7.55(1H,ddd,J=8.3,7.7,1.2Hz),7.42(1H,dd,J=8.3,7.7Hz).1-OAc:2.21(3H,s).14-OAc:2.18(3H,s).9-OAc:2.12(3H,s).15-OAc:2.11(3H,s).7-OAc:2.08(3H,s).8-OAc:2.06(3H,s);13C NMR(CDCl3,100MHz)δC198.4(C-5),142.1(C-7)133.4(C-13),130.4(C-6),122.1(C-12),89.4(C-15),84.9(C-1),78.8(C-9),76.4(C-3),71.8(C-14),68.7(C-8),56.5(C-4),44.2(C-2),40.2(C-11),38.4(C-10),28.5(C-19),20.1(C-18),16.1(C-20),13.7(C-17),12.1(C-16).3-OBz:165.5,129.7×2,129.5,128.5×2.8-OAc:170.4,20.9.7-OAc:170.0,20.8.1-OAc:169.9,20.7.9-OAc:169.6,22.0.14-OAc:168.9,20.7.15-OAc:167.6,20.1。
實施例20:YPTZ-33:(1S,2R,3S,4S,8R,9S,12S,13R,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基12,13-醚-假白欖烷-6E-烯的制備
取YPTZ-9,按照實施例18的方法可制備題述化合物。結構及數據如下:
YPTZ-33:[α]25D-38.7(c 0.31,CHCl3);UV(MeOH)λmax(logε)273(2.94),229(4.05)207(3.91)nm;IR(KBr)νmax33474,2976,2939,1759,1728,1375,1228,1073,1030,717cm-1;ESIMS m/z 797.3[M+Na]+;HREIMS m/z 797.3024[M+Na]+(calcd for C39H50O16Na,797.2991)。
1H NMR(CDCl3,400MHz)δH6.31(1H,s,H-8),6.18(1H,s,H-14),5.44(1H,d,J=4.5,4.2Hz,H-3),5.43(1H,d,J=11.4Hz H-1),5.19(1H,s,H-9),4.66(1H,dd,J=11.3,3.7Hz,H-5),4.20(1H,dd,J=4.1Hz,H-4),3.57(1H,d,J=3.4Hz,H-12),2.30(1H,m,H-2),1.33(1H,s,H-20),1.32(3H,s,H3-17),1.05(3H,s,H3-19),0.87(3H,d,J=6.8Hz,H3-16),0.85(3H,s,H3-18).3-OBz:7.96(2H,dd,J=8.0,1.0Hz),7.57(1H,ddd,J=8.0,7.7,1.0Hz),7.71(1H,dd,J=8.0,7.7Hz).7-OAc:2.16(3H,s).9-OAc:2.15(3H,s).1-OAc:2.10(3H,s).14-OAc:2.09(3H,s).15-OAc:2.08(3H,s),8-OAc:2.02(3H,s);13C NMR(CDCl3,100MHz)δC144.1(C-7),129.8(C-6),88.4(C-15),84.9(C-1),79.5(C-9),76.2(C-3),71.0(C-5),68.7(C-14),68.1(C-8),60.1(C-13),54.9(C-12),47.9(C-4),44.2(C-2),40.5(C-11),38.0(C-10),29.4(C-18),19.3(C-19),17.6(C-20),17.0(C-17),11.8(C-16).3-OBz:165.4,133.3,130.1,129.5×2,128.6×2.9-OAc:170.7,20.9.1-OAc:170.3,20.9.8-OAc:170.1,20.7.7-OAc:169.3,21.9.14-OAc:168.6,20.8.15-OAc:167.7,20.0。
實施例21:YPTZ-34:(1S,2R,3S,4R,5S,8R,9S,12S,13S,14R,15S)-1,7,8,9,14,15-六(乙酰基)-3-苯甲酰基-12-羥基-5,13-醚-假白欖烷-6E-烯的制備
取YPTZ-33(11mg),加入1%HCl甲醇溶液(5mL),在室溫下攪拌1h,減壓濃縮溶液后通過正相硅膠柱(洗脫劑為CH2Cl2)純化得到YPTZ-34(9mg)。結構及數據如下:
YPTZ-34:[α]25D-59.3(c 0.49,CHCl3);UV(MeOH)λmax(logε)273(2.77),230(3.94)205(3.83)nm;IR(KBr)νmax3522,2966,2936,1747,1247,1081,715cm-1;ESIMS m/z 797.3[M+Na]+;HREIMS m/z 797.3027[M+Na]+(calcd for C39H50O16Na,797.2991)。
1H NMR(CDCl3,400MHz)δH6.53(1H,s,H-8),5.91(1H,s,H-14),5.65(1H,d,J=3.6,3.5Hz,H-3),5.40(1H,d,J=9.4Hz,H-1),5.09(1H,s,H-9),4.79(1H,d,J=10.5Hz,H-5),4.15(1H,d,J=3.7Hz,H-12),3.31(1H,dd,J=10.5,3.5Hz,H-4),2.23(1H,m,H-2),2.20(1H,dd,J=15.9,6.9Hz,H-11a),1.36(1H,m,H-11b),1.34(1H,s,H-20),1.31(3H,s,H3-17),1.01(3H,d,J=6.9Hz,H3-16),0.92(3H,s,H3-18),0.85(3H,s,H3-19).3-OBz:8.00(2H,dd,J=7.2,1.2Hz),7.59(1H,ddd,J=7.8,7.2,1.2Hz),7.45(1H,dd,J=7.8,7.2Hz).14-OAc:2.36(3H,s).15-OAc:2.27(3H,s),9-OAc:2.11(3H,s).7-OAc:2.08(3H,s).1-OAc:2.06(3H,s).8-OAc:1.99(3H,s);13C NMR(CDCl3,100MHz)δC144.5(C-7),124.2(C-6),85.0(C-1),84.5(C-15),82.0(C-9),80.2(C-13),75.5(C-3),72.1(C-5),69.8(C-14),69.0(C-12),66.6(C-8),48.3(C-4),47.6(C-2),46.6(C-11),37.6(C-10),29.0(C-19),18.3(C-18),17.8(C-20),14.4(C-17),12.2(C-16).3-OBz:165.6,133.4,129.7,129.5×2,128.5×2.14-OAc:173.2,21.2.9-OAc:170.1,20.8.8-OAc:170.0,20.5.15-OAc:169.8,22.2.1-OAc:169.4,20.0.7-OAc:168.3,20.9。
實施例22:(1S,2R,3S,4R,5S,8R,9S,12S,13S,14R,15S)-1,7,8,9,12,14,15-七(乙酰基)-3-苯甲酰基-5,13-醚-假白欖烷-6E-烯的制備
取YPTZ-34,按照實施例10的方法可制備題述化合物。結構及數據如下:
YPTZ-35:[α]25D-100(c 0.04,CHCl3);UV(MeOH)λmax(logε)273(3.26),228(4.22)208(4.18)nm;IR(KBr)νmax2958,2917,1747,1460,1377,1247,1229,1085,1022cm-1;ESIMS m/z839.3[M+Na]+;HREIMS m/z 839.3140[M+Na]+(calcd for C41H52O17Na,839.3097)。
1H NMR(CDCl3,400MHz)δH6.58(1H,s,H-8),5.93(1H,s,H-14),5.64(1H,d,J=3.5,3.1Hz,H-3),5.44(1H,d,J=9.3Hz,H-1),5.43(1H,m,H-12),5.12(1H,s,H-9),4.82(1H,d,J=10.4Hz,H-5),3.32(1H,dd,J=10.4,3.1Hz,H-4),2.31(1H,m,H-11a),1.42(1H,s,H-20),1.32(3H,s,H3-17),1.05(3H,s,H3-18),0.98(3H,d,J=6.9Hz,H3-16),0.82(3H,s,H3-19).3-OBz:8.00(2H,dd,J=7.2,1.2Hz),7.59(1H,ddd,J=7.7,7.2,1.2Hz),7.45(1H,dd,J=7.7,7.2Hz).1-OAc:2.29(3H,s).8-OAc:2.19(3H,s).9-OAc:2.12(3H,s).7-OAc:2.08(3H,s).12-OAc:2.05(3H,s).14-OAc:2.00(3H,s).15-OAc:1.95(3H,s);13C NMR(CDCl3,100MHz)δC144.7(C-7),123.7(C-6),84.9(C-1),84.7(C-15),81.9(C-9),79.5(C-13),75.4(C-3),72.0(C-5),70.6(C-12),67.5(C-14),66.7(C-8),48.2(C-4),47.5(C-2),46.1(C-11),38.2(C-10),29.0(C-20).19.1(C-18),18.9(C-20),14.5(C-17),12.2(C-16).3-OBz:165.6,133.4,129.7,129.5×2,128.5×2.9-OAc:170.3,20.8.15-OAc:170.1,21.5.14-OAc:169.9,20.9.8-OAc:169.8,21.2.12-OAc:169.7,20.5.1-OAc:169.6,22.3.7-OAc:168.1,20.0。
細胞培養:HepG2/ADR,MCF-7/ADR和HLF系使用添加10%小牛血清的RPMI-1640培養液(Gibco BRL,USA),培養于含5%C的飽和濕度為37℃恒溫培養箱中,耐藥細胞株HepG2/ADR,MCF-7/ADR分別在培養基中加入1.2μM的阿霉素維持其耐藥。
MTT實驗:采用文獻報道的方法(Carmichael J,DeGraff WG,Gazdar AF,Minna JD,Mitchell JB.Evaluation of a tetrazolium-based semiautomated colorimetric assay:assessment of chemosensitivity testing.Cancer Res.1987,47(4):936-942.),分別以5×103個/mL密度接種于96孔培養板中,加入各種濃度的測試樣品,在37℃,5%CO2,90%RH的條件下培養48h;然后加入20μL的MTT溶液,再孵育4h,平板離心,棄去上清,加150ul DMSO溶解MTT結晶,平板振蕩數分鐘后,以酶聯免疫檢測儀在570nm波長測吸光度OD值。
化合物對于HepG2/ADR細胞Rho123外排作用的測定:HepG2/ADR細胞以5×105個細胞每孔的密度接種于6孔板,加入所需濃度的樣品和10μM Rho123在37℃,孵育培養90min。孵育結束后,棄去含Rho123的培養液,用冰PBS洗2次以充分洗滌除去Rho123,并將細胞重懸于PBS中,采用流式細胞儀525nm分析,測定熒光強度。計算外排倍數=(樣品共同孵育的熒光值-空白)/(羅丹明熒光值-空白)。同時將Tariquidar和維拉帕米,作為陽性對照測定細胞內的熒光強度。
化合物對于HepG2/ADR細胞Rho123外排作用的測定:HepG2/ADR細胞以5×105個細胞每孔的密度接種于6孔板,加入所需濃度的樣品和10μM Rho123在37℃℃,孵育培養90min。孵育結束后,棄去含Rho123的培養液,用冰PBS洗2次以充分洗滌除去Rho123,并將細胞重懸于PBS中,采用流式細胞儀525nm分析,測定熒光強度。計算外排倍數=(樣品共同孵育的熒光值-空白)/(羅丹明熒光值-空白)。同時將Tariquidar和維拉帕米,作為陽性對照測定細胞內的熒光強度。
化合物對預防阿霉素誘導引起的腫瘤細胞實驗:HepG2/ADR和MCF-7/ADR 2細胞以5×103個細胞每孔的密度接種于96孔板,然后分別加入不同濃度的假白欖烷型二萜成分(100nM,200nM,or 500nM)預處理細胞1小時。預處理后加入一定濃度的阿霉素繼續培養48小時,空白對照為僅加入新鮮培養液的細胞孔。細胞處理結束后,4℃離心收集細胞,冰PBS洗2次以充分除去藥物殘留,用MTT檢測分析細胞毒。Tariquidar和維拉帕米依法處理做為陽性對照。逆轉倍數=IC50(ADR)/to IC50(Pgp inhibitor+ADR)。所有實驗至少平行兩次。
聯合阿霉素治療耐藥腫瘤細胞移植瘤裸鼠的體內研究實驗:雌性BALB/c裸小鼠,5周,由中山大學動物房提供。HepG2/ADR的細胞系使用添加10%小牛血清的RPMI-1640培養液,置于含5%二氧化氮飽和濕度的37℃恒溫培養箱中培養。細胞成熟后,懸浮于RPMI-1640培養液中,濃度為6.67×107cells/mL。在每只裸鼠的右側注射耐藥菌株1×107cells造模,當腫瘤長到約60mm3的時候,將動物隨機分成3組,每組7只,每0,3,6天按制定方案腹腔注射給藥:1組,阿霉素=5mg/kg;2組,待測化合物(10mg/kg)+阿霉素=5mg/kg;3組,對照=生理鹽水(含0.5%DMSO+5%15-羥基硬脂酸聚乙二醇酯),每天記錄裸鼠腫瘤大小和體重,腫瘤體積=A×B2/2,(A=瘤體長徑,B=瘤體寬徑),記錄各組小鼠自實驗開始自死亡的生存天數,最后治療結束,將裸鼠的腫瘤移除體外測量體積和重量。
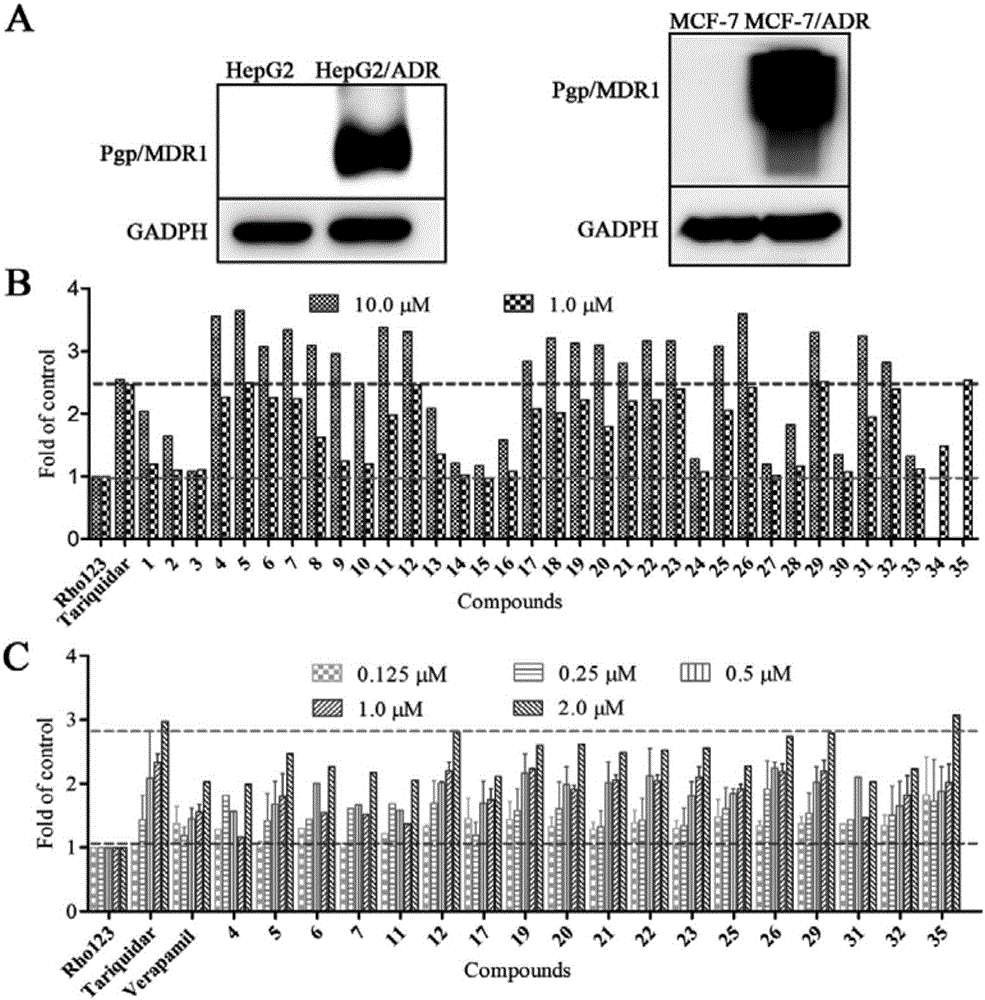
假白欖烷型二萜抑制Pgp介導的羅丹明123的外排活性測試:
按照上述細胞培養、化合物對于HepG2/ADR細胞Rho123外排作用的測定所述方法測試YPTZ-1~YPTZ-35抑制Pgp介導的羅丹明123的外排活性,結果見圖2。結果顯示大部分化合物顯示出很好的量藥效關系和各個濃度下的活性都優于相同濃度下的陽性對照verapamil。其中YPTZ-12,YPTZ-26,YPTZ-29和YPTZ-35在高濃度下(2.0μM)與tariquidar相當,而在低濃度(0.125μM)時優于tariquidar,所以該4個化合物被認為是潛在的Pgp活性藥物,是目前報道為止,具有類似結構中活性最好的化合物。
假白欖烷型二萜逆轉阿霉素肝癌細胞耐藥性研究:
按照上述細胞培養、MTT實驗的方法測定YPTZ-5,12,17,19-23,25,26,29和35對兩種耐藥菌株的細胞毒活性,結果如表1所示,所有的假白欖烷型二萜均未對兩種耐藥菌株顯示出明顯的細胞毒活性,同時陽性對照藥tariquidar顯示出明顯的細胞毒尤其是對MCF-7/ADR(IC50=13.1μM)。考慮到化合物有效濃度遠遠低于其細胞活性,所以這些化合物的細胞毒活性可以忽略不計。同時該類如表1所示,阿奇霉素對兩種耐藥菌株HepG2/ADR和MCF-7/ADR的細胞毒性,通過添加受試藥物假白欖烷型二萜,能明顯增強其活性對兩種細胞株分別的逆轉倍數達到了29-95倍和4-55倍,這種逆轉能力強于維拉帕米(11和6),而微弱于tariquidar(82和54倍)。
然后在200nM濃度下,受試化合物與不同濃度的阿霉素條件下,進行多藥耐藥逆轉實驗。其中化合物YPTZ-19,YPTZ-25和YPTZ-26對兩種細胞株均有很好的活性,進一步采用不同濃度:低濃度(100nM)和高濃度(500nM),對其檢測,盡管實驗結果沒有表現出明顯的量效關系,總結該類化合物的大量實驗數據可以看出,該些藥物的活性明顯強于維拉帕米,而和tariquidar相當,所以這些化合物具有開發成多藥耐藥抑制劑的潛力。
表1、化合物對耐藥菌株的細胞毒性和逆轉倍數
aIC50值(Pgp inhibitor+ADR)是平均值±至少兩個獨立實驗的標準方差(SD);e不可用;f未確定;b逆轉倍數為IC50(ADR)與IC50(Pgp inhibitor+ADR)的比值;c化合物對細胞的固有毒性,單個實驗的IC50值;dIC50(ADR)值是平均值±3個獨立實驗的標準方差(SD)s。
YPTZ-19,YPTZ-25,YPTZ-26對正常人肺成纖維細胞的細胞毒活性
實驗結果表明一些假白欖烷型化合物具有很好的逆轉和對耐藥菌株的低毒活性,但是它們在正常細胞菌株中是否低毒安全有效,發明人采用人肺成纖維細胞(HLF)作為測試細胞,如表2所示,在濃度高達100μM時,三個化合物均未見明顯的細胞毒,而與tariquidar相比較,后者具有明顯的細胞毒性,所以該結果也進一步證明這些化合物在治療作用優于tariquidar。
表2.YPTZ-19,YPTZ-25,YPTZ-26的細胞毒活性
aIC50值為平均值±兩個獨立實驗的標準誤(SD)。
肝微粒體實驗:為了檢測YPTZ-26在大鼠肝微粒體中的穩定,采用維拉帕米,tariquidar和睪丸酮(testosterone),作為陽性對照藥。根據文獻中的方法處理大鼠(Brunschweiger A,Iqbal J,Umbach F,Scheiff AB,Munkonda MN,Sevigny J,Knowles AF,Muller CE.Selective nucleoside triphosphate diphosphohydrolase-2(NTPDase2)inhibitors:Nucleotide mimeties derived fromuridine-5'-carboxamide.J.Med.Chem.2008,51(15):4518-4528.),得到肝微粒體,測定其蛋白濃度為31mg/mL。待測化合物,tariquidar,維拉帕米和睪丸酮溶解到乙腈中制成10mM的儲備液,然后樣品分別于大鼠肝微粒孵育使其最終濃度分別為1mg/mL的蛋白,100.0mM的PBS;3.0mM的MgCl2;1.0mM的NADPH;pH=7.4,樣品在不同孵育時間下(0,5,10,30,60,90,120,150,180min)在37℃的溫水中孵育,在孵育體系中分別加入0.5mL的冰乙腈終止其反應,并采用不加啟動子NADPH的體系,作為陰性對照,計入乙腈的樣品采用15000rpm,4℃,10分鐘,離心,用HPLC測定其峰面積,流動相純凈水(A),甲醇(B),采用梯度洗脫:0–9min,60%–100%B;9–11min,100%B;11–13min,100–60%B;13–15min,60%B,流速為1mL/min,計算其代謝穩定性。結果如表3所示,化合物維拉帕米,tariquidar和睪丸酮(testosterone)的肝微粒體代謝半衰期(T1/2)分別為25,210和2.5分鐘[14],YPTZ-26在大鼠肝微粒體中的半衰期時間為14.5小時,圖3,說明該化合物在大鼠肝體內具有很好的穩定性。
表3.YPTZ-26在大鼠肝微粒體中的代謝
a結果表示為平均值±至少3個獨立3重實驗的標準差。
YPTZ-26聯合阿霉素治療耐藥腫瘤細胞移植瘤裸鼠的體內研究
按上述的方法,評價YPTZ-26體內的活性。實驗結果表明:和對照組相比,聯合用藥組明顯的腫瘤體積明顯減小,如圖4A:對照組=144.48mm3,聯合用藥組=44.88mm3。同時聯合用藥的腫瘤質量也明顯下降:對照組=0.0563g,聯合用藥組=0.0209g,最終的腫瘤抑制率為62.89%(如圖4B-E)。值得注意的是只有阿霉素組別的7只裸鼠中,在12天內由于嚴重的毒副作用而出現死亡6只(圖4C)。而聯合用藥組(阿霉素+YPTZ-26)在3周以后依然有5只存活,說明該聯合用藥能夠延長裸鼠的生存壽命,而且腫瘤的體積在兩組比較中發現,聯合用藥組腫瘤體積明顯小于單獨給阿霉素組。以上結果表明,YPTZ-26可以作為一種有效的Pgp抑制劑,能夠有效的恢復MDR腫瘤敏感性化療不良反應的耐受劑量。
- 還沒有人留言評論。精彩留言會獲得點贊!
- 瑞香烷型二萜化合物pimelotide C的新用途的制作方法與工藝
- 大別山五針松的樹皮提取物及其制備方法和在制藥中的用途與流程
- 雷公藤二萜合酶TwCPS1在制備松香烷型二萜化合物中的應用的制造方法與工藝
- 雷公藤焦磷酸合酶TwCPS4及其制備松香烷型二萜化合物的應用的制造方法與工藝
- 新的抗炎松香烷型二萜糖苷tripterycosideC的制造方法與工藝
- 新的抗炎松香烷型二萜糖苷tripterycosideB的制造方法與工藝
- 新的抗炎松香烷型二萜糖苷tripterycosideA的制造方法與工藝
- 松香烷型二萜糖苷在制備抗炎藥物中的應用的制造方法與工藝
- 羥基化續隨子二萜烷型衍生物的轉化方法及其在制備抗腫瘤藥物中的用途與流程
- 一種新的半日花烷型二萜化合物及其制備方法和應用、藥物組合物及其應用與制造工藝